Kunshan Shuojing Photoelectric Technology Co., LTD
Mr. Jiang:18261685858
Mr. Jiang:13482461082
Email:gingerjzj@163.com
Adress:Room 809, Building 2, Jiayu International Business Plaza, Zhoushi Town, Kunshan City
Web address:shuojingcrystal.com
Sapphire crystal structure and morphology
Corundum crystals belong to the complex trigonal scalenohedron class D63d–R3C (L33L23PC) in triangular symmetry, with the following symmetry elements:
: Mirror rotation of the 6th axis (3rd reverse axis)
: 3 secondary axes perpendicular to it
: 3 planes of symmetry that are perpendicular to the 2nd axis and intersect along the highest axis
: center of symmetry
There are seven simple forms (faces) in this symmetry. According to the Bravais classification they have the following symbols: axicon (0001), hexagonal prism {1010} and {112 0}, complex hexagonal prism {hki0}, rhombohedron {h0h l}, hexagonal bipyramid {hh2h l} and bitriangular scalenohedron. Depending on the position of the corresponding hexagonal crystallographic axis, the rhombohedron may be "forward" {1011} or "reverse" {0111} [1].
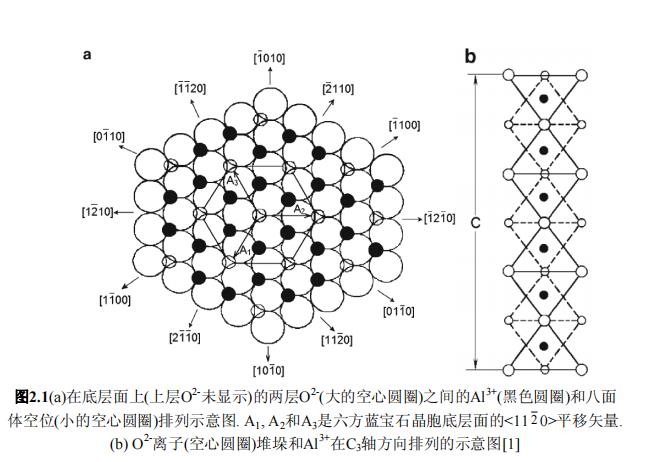
The lattice lattice of α-Al2O3 is composed of Al3+ and O2- ions. If the anion O2- is represented by a sphere, the lattice lattice adopts their hexagonal close-packed arrangement (Fig. 2.1). The cation Al3+ is located in the center without symmetry center In the lattice field (due to the deformation of the lattice lattice). These cations are located in close-packed octahedral vacancies adjacent to the O2- ions and occupy two-thirds of these vacancies. The octahedral vacancies are surrounded by six spheres. Taking the radius of each sphere as one unit, the radius of the sphere occupying the vacancy is 0.41 units. Due to the ionic radius ratio of O2- and Al3+ (1.40 and 0.57 Å, respectively), cations occupy the vacancies stacked by anions. Such cations will deform the lattice a little, but not exceed the stability limit of the octahedral position.
The coordination numbers of Al3+ and O2- are 6 and 4, respectively. The three O2- ions above the octahedron are rotated 64.3º with respect to the three O2- ions below and lie on parallel planes 2.164 Å apart from each other (Fig. 2.2a). The zui close-packed deformation is related to the difference between Al3+ size and octahedral position, and is confirmed by the fact that the octahedron consists of triangles of oxygen ions of different sizes (Fig. 2.2c) and the 64.3º turning angle between them Exceeds the characteristic angle value of the ideal stack (60º).
Kunshan Shuojing Optoelectronics Technology Co., Ltd. is involved in a wide range of new materials, and has complete preparation methods and means for functional ceramics and sapphire crystals, scintillation crystals, and laser crystals, forming optically transparent ceramics, sapphire optical windows, scintillation crystals, lasers and nonlinear crystals Master's four series of high-tech products